Is The Endoplasmic Reticulum In Plant Cells Or Animals
Introduction
The endoplasmic reticulum (ER) is an organelle with important functions in eukaryotic cells. It connects to other cellular compartments [e.chiliad., nucleus, Golgi apparatus, mitochondria, peroxisomes, plasma membrane (PM)] and, as one of the largest Ca2+ stores, participates in intracellular Ca2+ signaling. It is further involved in lipid and hormone biosynthesis (Staehelin, 1997; Sparkes et al., 2009; Lynes and Simmen, 2011). Importantly, the ER quality control (ER-QC) organisation mediates and monitors the processing and folding of secretory proteins destined for send to the PM, vacuole, or apoplast, identifies misfolded proteins and transfers them to the ER-associated degradation (ERAD) machinery (Vitale and Boston, 2008; Liu and Howell, 2010; Hüttner and Strasser, 2012). Amongst the proteins processed by the plant's ER-QC are important PM-resident proteins involved in adaptation to ecology stress, east.g., hormone or immune receptors (Saijo, 2010). ER integrity is central to proper function of cells and whole organisms. Especially under stress atmospheric condition, any impairment of ER function can result in disturbed institute development and constitute immunity (Wang et al., 2005; Vitale and Boston, 2008; Saijo, 2010).
Regulation of ER Integrity and ER Stress Signaling in Eukaryotes
Protein folding demand and capacities in the ER are normally in equilibrium. However, responses to environmental stresses create an increased requirement for secreted proteins. If this demand exceeds the ER-QC working capacity, unfolded proteins accrue in the ER, which the jail cell senses every bit ER stress. Prolonged ER stress impairs ER function and thus threatens cellular integrity. Chemicals, such as the North-glycosylation inhibitor tunicamycin (TM) or the reducing amanuensis dithiothreitol (DTT), which inhibits the formation of disulfide bonds, are widely used to induce and examine ER stress (Martínez and Chrispeels, 2003; Kamauchi et al., 2005; Vitale and Boston, 2008; Liu and Howell, 2010).
In animals, mainly three ER membrane proteins institute the cell's ER stress surveillance system: the blazon I transmembrane protein kinase/endoribonuclease inositol-requiring enzyme 1 (IRE1 α and β), the blazon I transmembrane protein kinase RNA-like ER kinase (PERK), and the type Two transmembrane basic leucine-attachment (bZIP) domain-containing activating transcription factor 6 (ATF6). In yeast cells, IRE1 is the only ER stress sensor (Mori, 2009). Under non-stressed conditions, luminal parts of these ER stress sensors bind to luminal bounden proteins (BiPs), which keeps the sensors in an inactive state. If unfolded proteins accumulate, BiPs disconnect from ER stress sensors to mediate processing of unfolded proteins. In one case liberated, ER stress sensors initiate different adaptive signaling cascades divers as unfolded poly peptide response (UPR) to re-establish proper ER function. The UPR enhances the synthesis of antioxidants and ER-QC members, attenuates translation, suppresses expression of secretory genes, and elevates ERAD of unfolded proteins (Schröder, 2006, 2008; Liu and Howell, 2010; Hetz, 2012;Higa and Chevet, 2012; Jäger et al., 2012). Figure 1A summarizes processes involved in UPR activation by the three ER stress sensors in animals. BiP release allows ATF6 translocation to the Golgi apparatus, where its cytosolic function (cATF6), a functional bZIP transcription cistron, is cleaved off by serine proteases S1P and S2P, a process called regulated intramembrane proteolysis (RIP). cATF6 and then enters the nucleus and promotes transcription of UPR genes and the bZIP transcription gene XBP1 (Yoshida et al., 2001). Upon BiP release, IRE1 oligomerizes and activates its endoribonuclease domain, leading to the anarchistic splicing of a 26 nucleotide intron out of XBP1 or its yeast counterpart HAC1, which allows the resulting proteins to enter the nucleus (Mori, 2009; Walter and Ron, 2011; Hetz, 2012). Phosphorylation by the PERK kinase activates the eukaryotic translation initiation cistron eIF2α, which attenuates translation but selectively promotes the translation of the transcription gene ATF4 (Harding et al., 2000). Eventually, ATF4, ATF6, and XBP1 (HAC1) elevate transcription of UPR genes (Mori, 2009; Walter and Ron, 2011; Hetz, 2012).
Figure 1
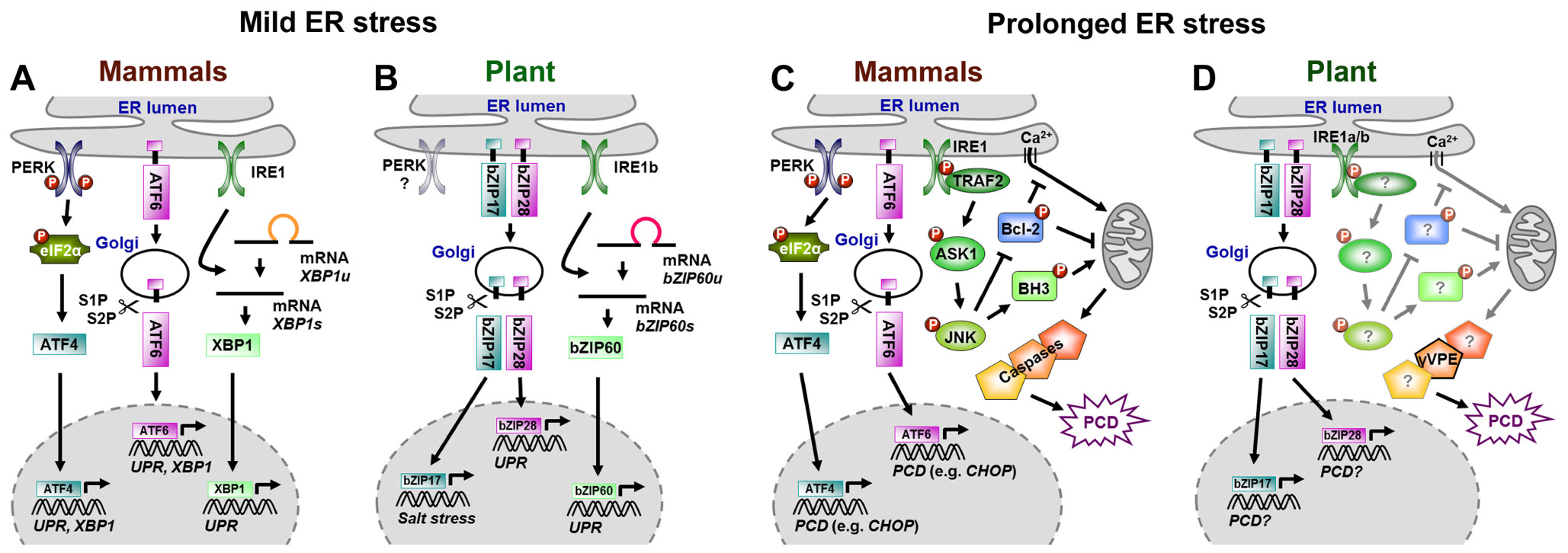
FIGURE 1. Signaling in mammals and plants under mild (A,B) and prolonged ER stress (C,D). Models indicate overlaps and differences in ER stress signaling. Conservation in mammalian (A) and plant (B) UPR signaling in response to balmy ER stress. Various components involved in mammalian ER-PCD signaling under prolonged ER stress have been identified (C), whereas plant ER-PCD signaling is almost unknown (D). Question marks (in D) indicate postulated orthologs or structural homologs of found ER-PCD signaling. XBP1u/bZIP60u, unspliced mRNA; XBP1s/bZIP60s, spliced mRNA.
In plants, the ER-QC and ER stress responses are apparently conserved as suggested by sequence homologies found in Arabidopsis for members of the ER translocon and oligosaccharyltransferase complexes too every bit for UPR and ERAD components (Liu and Howell, 2010). Further, transcripts of genes encoding proteins of the ER-QC machinery [e.g., chaperones BiPs, CALRETICULINs (CRTs), CALNEXINs (CNXs) or Protein DISULFIDE ISOMERASEs (PDIs)], or the ERAD pathway are induced by ER stress (Jelitto-Van Dooren et al., 1999; Leborgne-Castel et al., 1999; Koizumi et al., 2001; Martínez and Chrispeels, 2003; Kamauchi et al., 2005; Lu and Christopher, 2008; Su et al., 2011; Hüttner and Strasser, 2012). Putative constitute ER stress sensors and signaling components accept been identified (Effigy 1B), even so, except for IRE, corresponding constitute proteins do non show sequence just structural or functional homology (Koizumi et al., 2001; Liu and Howell, 2010). Arabidopsis possesses at least ii IRE1-like proteins, while only one homolog is nowadays in rice (Oryza sativa). AtIRE1a, AtIRE1b, and OsIRE1 harbor all structural features of yeast and mammalian IRE1. AtIRE1a and OsIRE1 are capable of autotransphosphorylation, and the putative ER stress sensor domain of AtIRE1a, AtIRE1b, and OsIRE1 can functionally supersede that of yeast IRE1 (Koizumi et al., 2001; Noh et al., 2002; Okushima et al., 2002). In that location are at least three ER-resident transmembrane bZIP transcription factors in Arabidopsis, which are involved in ER stress responses, AtbZIP17, AtbZIP28, and AtbZIP60 (Urade, 2009; Liu and Howell, 2010). Atbzip mutants practise not display morphological or developmental differences under not-stress conditions, but are more than sensitive to salt stress (Atbzip17, Liu et al., 2007b), rut (Atbzip28, Gao et al., 2008), or DTT treatment (Atbzip60, Humbert et al., 2012). The expression of salt stress responsive genes is impaired in Atbzip17 mutants (Liu et al., 2007b) as is the induction of canonical UPR genes in Atbzip28 and Atbzip60 mutants afterward TM treatment (Iwata and Koizumi, 2005a; Liu et al., 2007a; Iwata et al., 2008; Lu and Christopher, 2008; Tajima et al., 2008). Similar to ATF6 in mammals, AtbZIP17 and AtbZIP28 possess canonical S1P cleavage sites and are activated by a RIP-like procedure upon ER stress (Liu et al., 2007a,b, 2008a; Gao et al., 2008; Tajima et al., 2008; Che et al., 2010). RIP of AtbZIP17 and AtbZIP28 requires passage through the Golgi apparatus, where cleavage past the subtilisin-like serine protease AtS1P and subsequent processing by the metalloprotease AtS2P accept place (Liu et al., 2007a,b; Che et al., 2010; Srivastava et al., 2012). How these bZIPs sense ER stress and how Golgi transition is mediated, is not clear. However, TM treatment patently promotes the interaction of AtbZIP28 with the small-scale GTPase SAR1b and the guanidine commutation factor SEC12, which are putatively involved in coat protein complex Ii (COPII) vesicle formation during ER-to-Golgi transport (Srivastava et al., 2012). AtbZIP60 lacks a canonical S1P cleavage site and its activation is independent of S1P and S2P (Iwata et al., 2008). Similar to mammalian XBP1 and yeast HAC1, recent studies in Arabidopsis and rice revealed unconventional splicing of a 23 nucleotide intron from the AtbZIP60 mRNA past AtIRE1b or AtIRE1a, and a 20 nucleotide intron from its rice orthologOsbZIP50/OsbZIP74 mRNA past OsIRE1, e.g., after TM or salicylic acid (SA) treatment. This leads to a frame shift that removes the transmembrane domain of the new proteins and allows nuclear entrance (Deng et al., 2011; Nagashima et al., 2011; Hayashi et al., 2012; Humbert et al., 2012; Lu et al., 2012; Moreno et al., 2012). In that location are no obvious PERK homologs in Arabidopsis (Koizumi et al., 2001; Urade, 2009).
ER Stress AS Initiator of Programed Cell Expiry
The UPR is supposed to ensure cell survival. However, under prolonged or severe ER stress, mammalian cells activate an apoptosis-like programed cell expiry (ER-PCD) to eliminate damaged cells from stressed organisms (Schröder, 2006; Hetz, 2012; Jäger et al., 2012). The ER stress sensors ATF6, PERK, and IRE1 are fundamental regulators of this process every bit well (Figure 1C), although it is unclear how they perceive and differentiate signals to switch from UPR to apoptosis. ER-PCD obviously merges with other apoptosis pathways, involving enhanced generation of reactive oxygen species (ROS), and apoptosis-promoting Ca2+ signaling at ER and mitochondria (Chakrabarti et al., 2011; Gorman et al., 2012; Hetz, 2012; Jäger et al., 2012). The induction of the pro-apoptotic bZIP transcription factor CHOP (C/EBP-homologs poly peptide) by ATF6 and PERK/ATF4 during ER-PCD evidently is about relevant. CHOP down-regulates anti-apoptotic proteins (due east.g., BCL-two), only induces members of the pro-apoptotic (BH3)-merely protein family, e.one thousand., BIM (BCL-two-INTERACTING MEDIATOR OF CELL DEATH) or GADD34 (GROWTH ARREST AND DNA Damage-INDUCIBLE 34; Gorman et al., 2012; Hetz, 2012; Jäger et al., 2012). In add-on, IRE1 activates ER-PCD by interacting with TRAF2 (TUMOR NECROSIS Factor RECEPTOR-ASSOCIATED FACTOR 2; Gorman et al., 2012; Jäger et al., 2012). This initiates consecutive phosphorylation of ASK1 (APOPTOSIS SIGNAL-REGULATING KINASE 1) and JNK (JUN Northward-Last KINASE). Phosphorylation by JNK inactivates anti-apoptotic regulators such as BCL-two, but activates pro-apoptotic BH3-merely proteins such equally BIM or BID (BH3-interacting domain death agonist). BH3-only proteins promote the jail cell death activation-related oligomerization and translocation of BAX and BAK to the mitochondrial membrane, followed by cytochrome c release and caspase activation for execution of apoptosis. BCL-ii-dependent regulation of Catwo+ homeostasis of the ER likewise affects permeability transition and apoptosis signaling at mitochondria (Chakrabarti et al., 2011; Gorman et al., 2012; Hetz, 2012). BAX and BAK themselves can interact with IRE1 and promote its ability to activate ASK1 and JNK, processes that are plain blocked by the prison cell survival protein BI-1 (BAX INHIBITOR-1; Bailly-Maitre et al., 2009; Lisbona et al., 2009). Dynamic differential interactions with pro- and anti-apoptotic proteins modulated by the intensity and elapsing of ER stress signals might regulate separate functions of IRE1, and timely coordinated on- and offset of ATF6, PERK, and IRE1 signaling may play a decisive role in determining jail cell fate. In such a scenario, ER stress would initially actuate the adaptive UPR via IRE1-mediated splicing of XBP1. However, downward-regulation of the IRE1/XBP1 co-operative upon prolonged ER stress may give rise to pro-apoptotic IRE1/TREF2/ASK1/JNK, RIDD, and/or PERK signaling (Gorman et al., 2012; Hetz, 2012). Autophagy is further suggested to abolish ER stress in yeast and mammals every bit it might back up the removal of unfolded proteins (Bernales et al., 2006). Here, the PERK-elF2α-ATF4 and IRE/TRAF2/JNK pathways might connect autophagy to ER stress via the BECLIN1-BCL2 interaction and the induction of autophagy genes, respectively. Although ER stress-associated autophagy is thought to have a cytoprotective function, other studies advise a office in ER-PCD. However, regulators of this cell death pathway and its link to ER stress are currently unknown (Verfaillie et al., 2010; Aronson and Davies, 2012).
As in animal cells, prison cell expiry follows induction of UPR in TM-treated plants (Zuppini et al., 2004; Iwata and Koizumi, 2005b; Watanabe and Lam, 2008; Ishikawa et al., 2011). The molecular basis of plant ER-PCD and the part of institute bZIPs therein are largely unknown (Figure 1D). Yet, regulation of ER-PCD seems to be partially conserved beyond kingdoms, equally Arabidopsis BI-ane (AtBI-1) is involved in brake of ER-PCD in Arabidopsis as well (Watanabe and Lam, 2008; Ishikawa et al., 2011). AtBI-1 is AtbZIP60-dependently up-regulated in response to TM (Kamauchi et al., 2005; Iwata et al., 2008; Watanabe and Lam, 2008). AtBI-one-mediated inhibition of ER-PCD in Arabidopsis is likely un-related to UPR modification, but rather to the suppression of ER-dependent ROS production or regulation of jail cell death associated ER Ca2+ homeostasis (Watanabe and Lam, 2008, 2009). In Arabidopsis, a Gβ subunit of an ER-resident heterotrimeric GTP-binding protein, AGB1, might be involved in the promotion of ER-PCD (Wang et al., 2007; Chen and Brandizzi, 2012). Disturbed ER protein retention later on silencing of NbERD2a/NbERD2b interferes with ER-QC and reduces ER stress alleviation, resulting in enhanced PCD in response to bacterial pathogens (Xu et al., 2012). New insights into the office of vacuolar processing enzymes with caspase1-like activities in the execution of ER-PCD come up from Qiang et al. (2012). These studies demonstrate the dependence of the mutualistic fungus Piriformospora indica on ER-PCD for successful Arabidopsis root colonization. P. indica induces ER stress but suppresses the adaptive UPR pathway. Consequently, the P. indica-induced ER stress triggers a vacuolar cell expiry pathway whose execution depends on γ VACUOLAR PROCESSING ENZYME (γVPE). This ER-PCD can be phenocopied past the application of TM to Arabidopsis roots. The analyses further show that γVPE is responsible for enhanced VPE and caspase 1-like activities during TM- and P. indica-induced ER-PCD (Qiang et al., 2012).
ER – Executor of Establish Immunity and Putative Target of Pathogen Effectors
Plants ward off pathogens by a multi-layered immune system. PM localized blueprint recognition receptors (PRRs) detect conserved molecules, and then-chosen microbe-associated molecular patterns (MAMPs), of invading microbes. Well-characterized PRRs are FLAGELLIN-SENSING 2 (FLS2), which recognizes bacterial flagellin, the ELONGATION-Cistron TU (EF-Tu) RECEPTOR (EFR), which detects bacterial EF-Tu, and the chitin receptors CHITIN ELICITOR Bounden PROTEIN (CEBiP) and CHITIN ELICITOR RECEPTOR KINASE (CERK; Monaghan and Zipfel, 2012). MAMP perception by these PRRs initiates allowed signaling pathways, defined as MAMP-triggered immunity (MTI), which involve Ca2+ fluxes beyond the PM, a rapid production of ROS, the activation of mitogen-activated protein kinase cascades and WRKY transcription factors, somewhen resulting in the induction of defense force mechanisms including callose depositions and the synthesis of antimicrobial pathogenesis-related (PR) proteins (Jones and Dangl, 2006; Boller and Felix, 2009). Successful pathogens accept evolved effector molecules to suppress MTI. Found RESISTANCE (R) proteins specifically recognize pathogen effectors or their activities and initiate effector-triggered immunity (ETI), typically involving hypersensitive response (HR)-related PCD (Chisholm et al., 2006; Jones and Dangl, 2006). The ER participates in found innate immunity in several means. Firstly, immunity depends on the secretory apparatus for the product of immune proteins (Wang et al., 2005; Nekrasov et al., 2009; Saijo et al., 2009). NONEXPRESSOR OF PR GENES 1 (NPR1), the master regulator of SA-dependent systemic acquired resistance (SAR), coordinately controls the up-regulation of PR genes and genes encoding proteins of the secretory pathway during SAR (Wang et al., 2005). Secondly, synthesis and proper part of PRRs (e.g., EFR) rely on Due north-glycosylation and the ER-QC system, which involves staurosporine and temperature sensitive-3a (STT3A), glucosidase II, the H/KDEL receptor ERD2b, the UDP-glucose:glycoprotein glucosyltransferase (UGGT)/CRT3 cycle and the stromal prison cell-derived factor-2 (SDF2)/ERdj3B/BiP complex (Li et al., 2009; Lu et al., 2009; Nekrasov et al., 2009; Saijo et al., 2009; Saijo, 2010). Susceptibility of ER-QC mutants to pathogens differs qualitatively and quantitatively from that of efr mutants, suggesting the existence of EFR-independent just ER-QC-dependent immune response (Li et al., 2009; Nekrasov et al., 2009; Saijo et al., 2009). Meanwhile, a number of membrane-localized immune receptors take been identified, whose functions depend on ER-QC, amid them the rice PRR XA21 involved in resistance to Xanthomonas oryzae pv. oryzae (Park et al., 2010a,b), an induced receptor kinase (IRK), which is involved in N-mediated resistance of tobacco to tobacco mosaic virus (Caplan et al., 2009), and glycosylated Cf proteins, which confer race-specific resistance to the fungal pathogen Cladosporium fulvum (Liebrand et al., 2012). Similar to FLS2, the ER-QC disturbance does non affect CERK1 function in Arabidopsis (Li et al., 2009; Nekrasov et al., 2009). Even so, the rice homolog OsCERK1 seems to interact with a Hop/Sti1-Hsp90 chaperone complex for maturation in the ER prior to transport to the PM (Chen et al., 2010). ER-QC likewise monitors glycosylation and proper folding of some immunity-related Toll-like receptors (TLRs) that recognize MAMPs in animals (Yang et al., 2007). Interestingly, PRRs TLR4 and TLR2 actuate the IRE1α-XBP1 pathway to enhance secretion of certain proinflammatory cytokines in macrophages, and loss of XBP1 part impairs immunity against the bacterial pathogen Francisella tularensis (Martinon et al., 2010).
Induction of the ER-QC machinery accompanies synthesis of amnesty-associated proteins in plants (Jelitto-Van Dooren et al., 1999; Wang et al., 2005). Consequently, ER-QC mutants are more than susceptible to ER stress inducers and pathogens (Wang et al., 2005; Li et al., 2009; Lu et al., 2009; Nekrasov et al., 2009; Saijo et al., 2009). Similarly, proper execution of defense responses may rely on the induction of UPR genes. Recently, the heat-shock cistron-similar transcription gene TBF1 has been identified every bit of import transcriptional regulator of UPR genes, and Arabidopsis tbf1 mutants are impaired in the execution of SAR and EFR-mediated MTI (Pajerowska-Mukhtar et al., 2012). The Nicotiana benthamiana homolog of AtbZIP60, NbbZIP60, is induced in response to inoculation with avirulent Pseudomonas cichorii and required to arrest its growth (Tateda et al., 2008). Furthermore, AtIRE1a and AtIRE1b expression is pathogen-responsive, and both proteins are required for SA or pathogen-dependent splicing of AtbZIP60, expression of ER-QC genes, secretion of defence proteins and thus execution of SAR (Moreno et al., 2012).
Together, this underlines the functional importance of the ER in both MTI and ETI, and designates it as a potential effector target. Consequent with this, many viruses use host UPR by targeting ER stress sensors to enhance folding of viral proteins or to modulate immune responses in mammals (Ke and Chen, 2011; Qian et al., 2012). In tobacco, infection with White potato virus X or overexpression of a viral movement poly peptide induces bZIP60 and UPR genes possibly to suppress host cell expiry responses (Ye et al., 2011). In improver, Yamamoto et al. (2011) showed that ATF6β is part of mice immunity against the protozoan parasite Toxoplasma gondii. ROP18, a serine/threonine kinase, which is secreted into the host cell during infection, interacts with ATF6β and mediates its proteasome-dependent degradation. Thus, ATF6β constitutes a target for the T. gondii ROP18 virulence factor possibly to suppress UPR-mediated host defense. Likewise, the Salmonella enterica leucine-rich repeat (LRR) effector protein SlrP targets the host ER-QC member ERdj3. This supports infection as it leads to the accumulation of unfolded proteins eventually promoting host cell death (Bernal-Bayard et al., 2010). In Caenorhabditis elegans, the increased requirement of secreted proteins during the activation of allowed responses imposes ER stress to the organism itself, which requires XBP1-mediated UPR to avoid onset of ER-PCD (Richardson et al., 2010). Several bacterial toxins, e.g., Shiga toxin produced by enterohemorrhagic bacteria, tin can enter the ER and seem to initiate cell death through prolonged UPR signaling past activating ER stress sensors (Tesh, 2012).
Conclusions and Perspective
As production site of antimicrobial proteins and of immune signaling components, the ER functions as central regulator in the execution of allowed responses in plants and animals. Therefore, the disturbance of ER integrity is certainly of primary relevance for pathogens to attain host cell infection. Plants farther rely on proper ER function and likely ER membrane localized stress sensors for accommodation to abiotic stress such as common salt or heat stress (Liu et al., 2008a,b, 2011; Che et al., 2010; Cui et al., 2012). Taken together, the improvement of plant UPR in order to maintain ER homeostasis under unfavorable conditions may increase plant adaptability to biotic and abiotic stress, which bears a potential to raise crop yield and yield stability.
Disharmonize of Interest Statement
The authors declare that the inquiry was conducted in the absenteeism of any commercial or fiscal relationships that could be construed as a potential conflict of involvement.
Acknowledgments
We kindly thank the High german Research Council (Deutsche Forschungsgemeinschaft) for fiscal support through grants to Ruth Eichmann (EI835/1-1) and Patrick Schäfer (SCHA1444/three-iii).
References
Bailly-Maitre, B., Belgardt, B. F., Hashemite kingdom of jordan, Southward. D., Coornaert, B., von Freyend, M. J., Kleinridders, A., Mauer, J., Cuddy, Thou., Kress, C. L., Willmes, D., Essig, M., Hampel, B., Protzer, U., Reed, J. C., and Brüning, J. C. (2009). Hepatic Bax inhibitor-ane inhibits IRE1α and protects from obesity-associated insulin resistance and glucose intolerance. J. Biol. Chem. 285, 6198–6207.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Bernal-Bayard, J., Cardenal-Muñoz, E., and Ramos-Morales, F. (2010). The Salmonella type Three secretion effector, Salmonella leucine-rich repeat protein (SlrP), targets the human chaperone ERdj3. J. Biol. Chem. 285, 16360–16368.
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Bernales, S., McDonald, K. L., and Walter, P. (2006). Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. iv, e423. doi: x.1371/journal.pbio.0040423
Pubmed Abstruse | Pubmed Total Text | CrossRef Full Text
Boller, T., and Felix, G. (2009). A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. lx, 379–406.
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Caplan, J. L., Zhu, X., Mamillapalli, P., Marathe, R., Anandalakshmi, R., and Dinesh-Kumar, Due south. P. (2009). Induced ER chaperones regulate a receptor-similar kinase to mediate antiviral innate immune response in plants. Cell Host Microbe 6, 457–469.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Che, P., Bussell, J. D., Zhou, Due west., Estavillo, G. One thousand., Pogson, B. J., and Smith, Southward. M. (2010). Signaling from the endoplasmic reticulum activates brassinosteroid signaling and promotes acclimation to stress in Arabidopsis. Sci. Indicate. 3, ra69.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Chen, 50., Hamada, Due south., Fujiwara, M., Zhu, T., Thao, N. P., Wong, H. 50., Krishna, P., Ueda, T., Kaku, H., Shibuya, N., Kawasaki, T., and Shimamoto, K. (2010). The Hop/Sti1-Hsp90 chaperone complex facilitates the maturation and transport of a PAMP receptor in rice innate immunity. Cell Host Microbe seven, 185–196.
Pubmed Abstruse | Pubmed Full Text | CrossRef Total Text
Cui, F., Liu, L., Zhao, Q., Zhang, Z., Li, Q., Lin, B., Wu, Y., Tang, Southward., and Xie, Q. (2012). Arabidopsis ubiquitin conjugase UBC32 is an ERAD component that functions in brassinosteroid-mediated salt stress tolerance. Establish Cell 24, 233–244.
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Deng, Y., Humbert, S., Liu, J. X., Srivastava, R., Rothstein, Due south. J., and Howell, S. H. (2011). Oestrus induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proc. Natl. Acad. Sci. U.Southward.A. 108, 7247–7252.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Gao, H., Brandizzi, F., Benning, C., and Larkin, R. M. (2008). A membrane-tethered transcription factor defines a branch of the estrus stress response in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 105, 16398–16403.
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Harding, H. P., Novoa, I., Zhang, Y., Zeng, H., Wek, R., Schapira, M., and Ron, D. (2000). Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell six, 1099–1108.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Hayashi, S., Wakasa, Y., Takahashi, H., Kawakatsu, T., and Takaiwa, F. (2012). Betoken transduction past IRE1-mediated splicing of bZIP50 and other stress sensors in the endoplasmic reticulum stress response of rice. Found J. 69, 946–956.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Humbert, Due south., Zhong, S., Deng, Y., Howell, S. H., and Rothstein, S. J. (2012). Alteration of the bZIP60/IRE1 pathway affects constitute response to ER stress in Arabidopsis thaliana. PLoS ONE 7, e39023. doi: ten.1371/periodical.pone.0039023
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Ishikawa, T., Watanabe, Due north., Nagano, 1000., Kawai-Yamada, M., and Lam, Due east. (2011). Bax inhibitor-1: a highly conserved endoplasmic reticulum-resident cell expiry suppressor. Jail cell Decease Differ. xviii, 1271–1278.
Pubmed Abstract | Pubmed Total Text | CrossRef Total Text
Iwata, Y., Fedoroff, North. V., and Koizumi, N. (2008). Arabidopsis bZIP60 is a proteolysis-activated transcription factor involved in the endoplasmic reticulum stress response. Plant Cell twenty, 3107–3121.
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Iwata, Y., and Koizumi, N. (2005a). An Arabidopsis transcription factor, AtbZIP60, regulates the endoplasmic reticulum stress response in a way unique to plants. Proc. Natl. Acad. Sci. U.S.A. 102, 5280–5285.
Pubmed Abstruse | Pubmed Full Text | CrossRef Total Text
Jäger, R., Bertrand, M. J., Gorman, A. One thousand., Vandenabeele, P., and Samali, A. (2012). The unfolded protein response at the crossroads of cellular life and death during endoplasmic reticulum stress. Biol. Jail cell 104, 259–270.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Jelitto-Van Dooren, E. P., Vidal, South., and Denecke, J. (1999). Anticipating endoplasmic reticulum stress. A novel early on response before pathogenesis-related gene induction. Plant Cell eleven, 1935–1944.
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Ke, P. Y., and Chen, Due south. Southward. (2011). Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro. J. Clin. Invest. 121, 37–56.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Koizumi, N., Martinez, I. M., Kimata, Y., Kohno, Yard., Sano, H., and Chrispeels, Grand. J. (2001). Molecular label of two Arabidopsis Ire1 homologs, endoplasmic reticulum-located transmembrane protein kinases. Found Physiol. 127, 949–962.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Leborgne-Castel, N., Jelitto-Van Dooren, Due east. P. Due west. M., Crofts, A. J., and Denecke, J. (1999). Overexpression of BiP in tobacco alleviates endoplasmic reticulum stress. Plant Cell 11, 459–469.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Li, J., Zhao-Hui, C., Batoux, Yard., Nekrasov, V., Roux, Thousand., Chinchilla, D., Zipfel, C., and Jones, J. D. (2009). Specific ER quality command components required for biogenesis of the constitute innate immune receptor EFR. Proc. Natl. Acad. Sci. UsaA. 106, 15973–15978.
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Liebrand, T. Westward., Smit, P., Abd-El-Haliem, A., de Jonge, R., Cordewener, J. H., America, A. H., Sklenar, J., Jones, A. Grand., Robatzek, S., Thomma, B. P., Tameling, Due west. I., and Joosten, M. H. (2012). ER-quality control chaperones facilitate the biogenesis of Cf receptor-similar proteins involved in pathogen resistance of tomato. Constitute Physiol. 159, 1819–1833.
Pubmed Abstract | Pubmed Total Text | CrossRef Total Text
Lisbona, F., Rojas-Rivera, D., Thielen, P., Zamorano, S., Todd, D., Martinon, F., Glavic, A., Kress, C., Lin, J. H., Walter, P., Reed, J. C., Glimcher, L. H., and Hetz, C. (2009). BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1α. Mol. Prison cell 33, 679–691.
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Liu, J. 10., Srivastava, R., Che, P., and Howell, Southward. H. (2007a). An endoplasmic reticulum stress response in Arabidopsis is mediated by proteolytic processing and nuclear relocation of a membrane-associated transcription factor, bZIP28. Constitute Prison cell 19, 4111–4119.
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Liu, J. Ten., Srivastava, R., Che, P., and Howell, S. H. (2007b). Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J. 51, 897–909.
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Liu, J. 10., Srivastava, R., Che, P., and Howell, S. H. (2008a). Salt stress signaling in Arabidopsis thaliana involves a membrane-bound transcription gene AtbZIP17 as a betoken transducer. Plant Signal. Behav.3, 56–57.
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Liu, J. Ten., Srivastava, R., and Howell, Southward. H. (2008b). Stress-induced expression of an activated form of AtbZIP17 provides protection from salt stress in Arabidopsis. Constitute Cell Environ. 31, 1735–1743.
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Liu, Fifty., Cui, F., Li, Q., Yin, B., Zhang, H., Lin, B., Wu, Y., Xia, R., Tang, South., and Xie, Q. (2011). The endoplasmic reticulum-associated deposition is necessary for plant common salt tolerance. Cell Res. 21, 957–969.
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Lu, D. P., and Christopher, D. A. (2008). Endoplasmic reticulum stress activates the expression of a sub-grouping of protein disulfideisomerase genes and AtbZIP60 modulates the response in Arabidopsis thaliana. Mol. Genet. Genomics 280, 199–210.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Lu, S. J., Yang, Z. T., Sun, L., Sun, L., Song, Z. T., and Liu, J. 10. (2012). Conservation of IRE1-regulated bZIP74 mRNA unconventional splicing in rice (Oryza sativa L.) involved in ER stress responses. Mol. Constitute v, 504–514.
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Lu, 10., Tintor, N., Mentzel, T., Kombrink, E., Boller, T., Robatzek, Southward., Schulze-Lefert, P., and Saijo, Y. (2009). Uncoupling of sustained MAMP receptor signaling from early outputs in an Arabidopsis endoplasmic reticulum glucosidase II allele. Proc. Natl. Acad. Sci. U.S.A. 106, 22522–22527.
Pubmed Abstruse | Pubmed Full Text | CrossRef Total Text
Lynes, E. M., and Simmen, T. (2011). Urban planning of the endoplasmic reticulum (ER): how diverse mechanisms segregate the many functions of the ER. Biochim. Biophys. Acta 1813, 1893–1905.
Pubmed Abstruse | Pubmed Full Text | CrossRef Total Text
Martínez, I. M., and Chrispeels, M. J. (2003). Genomic assay of the unfolded protein response in Arabidopsis shows its connection to of import cellular processes. Plant Cell 15, 561–576.
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Moreno, A. A., Mukhtar, M. S., Blanco, F., Boatwright, J. Fifty., Moreno, I., Jordan, M. R., Chen, Y., Brandizzi, F., Dong, X., Orellana, A., and Pajerowska-Mukhtar, K. M. (2012). IRE1/bZIP60-mediated unfolded protein response plays distinct roles in plant immunity and abiotic stress responses. PLoS ONE 7, e31944. doi: 10.1371/periodical.pone.0031944
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Nagashima, Y., Mishiba, Chiliad., Suzuki, Eastward., Shimada, Y., Iwata, Y., and Koizumi, North. (2011). Arabidopsis IRE1 catalyses unconventional splicing of bZIP60 mRNA to produce the agile transcription factor. Sci. Rep. 1, 29.
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Nekrasov, V., Li, J., Batoux, M., Roux, Yard., Chu, Z. H., Lacombe, Due south., Rougon, A., Bittel, P., Kiss-Papp, M., Chinchilla, D., van Esse, H. P., Jorda, Fifty., Schwessinger, B., Nicaise, V., Thomma, B. P., Molina, A., Jones, J. D., and Zipfel, C. (2009). Control of the design-recognition receptor EFR by an ER protein complex in plant amnesty. EMBO J. 28, 3428–3438.
Pubmed Abstruse | Pubmed Total Text | CrossRef Total Text
Noh, South. J., Kwon, C. S., and Chung, W. I. (2002). Characterization of ii homologs of Ire1p, a kinase/endoribonuclease in yeast, in Arabidopsis thaliana. Biochim. Biophys. Acta 1575, 130–134.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Okushima, Y., Koizumi, North., Yamaguchi, Y., Kimata, Y., Kohno, K., and Sano, H. (2002). Isolation and characterization of a putative transducer of endoplasmic reticulum stress in Oryza sativa. Plant Cell Physiol. 43, 532–539.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Pajerowska-Mukhtar, One thousand. Thou., Wang, W., Tada, Y., Oka, N., Tucker, C. Fifty., Fonseca, J. P., and Dong, X. (2012). The HSF-similar transcription cistron TBF1 is a major molecular switch for plant growth-to-defense transition. Curr. Biol. 22, 103–112.
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Park, C. J., Bart, R., Chern, M., Canlas, P. E., Bai, W., and Ronald, P. C. (2010a). Overexpression of the endoplasmic reticulum chaperone BiP3 regulates XA21-mediated innate amnesty in rice. PLoS ONE 5, e9262. doi: 10.1371/journal.pone.0009262
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Qian, Z., Xuan, B., Chapa, T. J., Gualberto, N., and Yu, D. (2012). Murine cytomegalovirus targets transcription cistron ATF4 to exploit the unfolded-protein response. J. Virol. 86, 6712–6723.
Pubmed Abstruse | Pubmed Total Text | CrossRef Total Text
Qiang, X., Zechmann, B., Reitz, Chiliad. U., Kogel, K. H., and Schäfer, P. (2012). The mutualistic fungus Piriformospora indica colonizes Arabidopsis roots past inducing an endoplasmic reticulum stress-triggered caspase-dependent prison cell death. Found Jail cell 24, 794–809.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Saijo, Y., Tintor, N., Lu, 10., Rauf, P., Pajerowska-Mukhtar, Grand., Häweker, H., Dong, X., Robatzek, S., and Schulze-Lefert, P. (2009). Receptor quality control in the endoplasmic reticulum for institute innate immunity. EMBO J. 28, 3439–3449.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Srivastava, R., Chen, Y., Deng, Y., Brandizzi, F., and Howell, S. H. (2012). Elements proximal to and within the transmembrane domain mediate the organelle-to-organelle motion of bZIP28 nether ER stress conditions. Plant J. 70, 1033–1042.
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Su, W., Liu, Y., Xia, Y., Hong, Z., and Li, J. (2011). Conserved endoplasmic reticulum-associated degradation arrangement to eliminate mutated receptor-like kinases in Arabidopsis. Proc. Natl. Acad. Sci. The statesA. 108, 870–875.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Tajima, H., Iwata, Y., Iwano, M., Takayama, S., and Koizumi, N. (2008). Identification of an Arabidopsis transmembrane bZIP transcription gene involved in the endoplasmic reticulum stress response. Biochem. Biophys. Res. Commun. 374, 242–247.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Tateda, C., Ozaki, R., Onodera, Y., Takahashi, Y., Yamaguchi, K., Berberich, T., Koizumi, N., and Kusano, T. (2008). NtbZIP60, an endoplasmic reticulum-localized transcription factor, plays a role in the defence response against bacterial pathogens in Nicotiana tabacum. J. Plant Res. 121, 603–611.
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text
Watanabe, N., and Lam, E. (2009). Bax inhibitor-1, a conserved cell expiry suppressor, is a cardinal molecular switch downstream from a multifariousness of biotic and abiotic stress signals in plants. Int. J. Mol. Sci. 10, 3149–3167.
Pubmed Abstract | Pubmed Total Text | CrossRef Full Text
Xu, G., Li, S., Xie, Grand., Zhang, Q., Wang, Y., Tang, Y., Liu, D., Hong, Y., He, C., and Liu, Y. (2012). Plant ERD2-similar proteins part as ER luminal protein receptors and participate in programmed cell death during innate immunity. Institute J. doi: 10.1111/j.1365-313X.2012.05053.x [Epub alee of impress].
Pubmed Abstract | Pubmed Total Text | CrossRef Total Text
Yamamoto, 1000., Ma, J. S., Mueller, C., Kamiyama, N., Saiga, H., Kubo, Due east., Kimura, T., Okamoto, T., Okuyama, Chiliad., Kayama, H., Nagamune, K., Takashima, S., Matsuura, Y., Soldati-Favre, D., and Takeda, K. (2011). ATF6β is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J. Exp. Med. 208, 1533–1546.
Pubmed Abstract | Pubmed Total Text | CrossRef Total Text
Yang, Y., Liu, B., Dai, J., Srivastava, P. Thousand., Zammit, D. J., Lefrancois, 50., and Li, Z. (2007). Estrus daze protein gp96 is a principal chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity 26, 215–226.
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Ye, C., Dickman, M. B., Whitham, South. A., Payton, M., and Verchot, J. (2011). The unfolded poly peptide response is triggered past a institute viral movement poly peptide. Plant Physiol. 156, 741–755.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T., and Mori, K. (2001). XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly agile transcription cistron. Prison cell 107, 881–891.
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text
Source: https://www.frontiersin.org/articles/10.3389/fpls.2012.00200/full
Posted by: burtonwintralmor.blogspot.com

0 Response to "Is The Endoplasmic Reticulum In Plant Cells Or Animals"
Post a Comment